Quantum biology is a new field that covers a vast territory of unexplored phenomena. The biophysical processes that are used by biological systems have been shown to utilize a number of quantum mechanical effects, but many more remain undiscovered or unrecognized. A good example of this current state of quantum biology is presented by ferritin, a ubiquitous iron storage protein that has a number of known quantum biological properties. Those quantum biological properties appear to be used by a number of diverse biological processes, yet very few scientists have conducted formal investigations into that aspect of ferritin in those systems. It is likely that many other quantum biological processes remain undiscovered or unrecognized, understanding the clues that point to ferritin's quantum biological functions might help to identify quantum biological properties of other biosystems.
Quantum mechanics (QM) describes the physical behavior of fundamental particles like electrons and photons as probability wave functions, as opposed to the classical understanding that those particles are discrete and localized objects like tiny billiard balls. QM was discovered ~100 years ago, and the scientists who discovered it believed that it was a necessary tool for the understanding of biological systems. Yet the biologists of that era did not understand QM, and the classical approaches used by biologists were “good enough” approximations to solve most of the problems in biology. In the years since the discovery of QM, slow progress has been made towards understanding how it applies to biological systems and processes.
Several important discoveries in the field of quantum biology have helped to shed light on how to approach the application of QM to biological properties – literally. The study of photosynthesis has led to a better understanding of how photon energy can be converted into stored chemical energy, after classical approaches failed to do so. The study of radical pairs has demonstrated one way that magnetic fields may be able to influence the behavior of plants and animals, which classical approaches are unable to explain. For some of these discoveries, the way that cells use pigment-protein complexes was found to be important, and using QM to analyze pigment-protein complexes was able to answer questions that classical approaches could not. These complex biostructures evolved within single cells over billions of years to utilize physical properties that are described by QM, and later in multicellular organisms as they evolved and increased in complexity. One valuable tool for understanding quantum biology is to look at questions that cannot be answered using classical approaches to cellular behavior, and to evaluate whether quantum biological mechanisms are involved with biological processes.
The quantum biological properties of pigment-protein complexes invited the question of whether such complexes in the brain might use QM. The most pronounced expression of pigment in the brain is neuromelanin, which is like melanin in many other biological systems in plants and animals. Melanin is a pi-conjugated polymer (which means that it has atoms with overlapping electron orbitals) that exhibits semiconductor-type electrical behavior. Further investigation identified the protein ferritin as part of a pigment-protein complex in catecholaminergic neurons with melanin that produce the powerful neurotransmitters dopamine and norepinephrine. Two of the neural nuclei that have high concentrations of ferritin and neuromelanin – the substantia nigra pars compacta (SNc) and the locus coeruleus (LC) – directly interface with cortical and sensory neurons across the brain, and provide dopamine and noradrenaline to the brain for action selection, a key function of consciousness. They are uniquely configured to integrate cognitively processed neural information as part of a global neuronal workspace using quantum biological electron transport and ferritin-melanin pigment protein complexes.
It has been shown that ferritin is required for photosynthesis in some plants (1). However, ferritin also has bioelectric, bio-magnetic and bio-optical properties that are independent of radical pairs or pigment-protein complexes, and those properties can answer questions about cells and diverse biological systems that classical approaches fail to, including cells and systems in the eye, in the ear, in myelin, and in macrophages that are found in the heart and throughout the body (2). While the study of the quantum biology of ferritin in these systems is in its infancy, the process by which these quantum biological properties ferritin were identified is provided here as a roadmap that could be used by others to investigate other quantum biophysical processes, and demonstrate how life evolves to use quantum biology.
The pre-biological discovery of ferritin’s quantum properties
Ferritin is a unique and unusual biological structure that is essentially a nanomachine – a machine that uses individual atoms to perform complex processes, and having dimensions that are measured in nanometers, one billionth of a meter. It is formed by protein subunits that self-assemble into a hollow shell, and which can oxidize water-soluble ferrous iron (Fe2+) into water-insoluble ferric iron (Fe3+). The ferric iron is stored as crystalline ferrihydrite ((Fe3+)2O3·0.5H2O), which forms particles known as superparamagnetic iron oxide nanoparticles (SPIONs). “Superparamagnetic” means they can be attracted to a magnet but are not magnetized. Ferritin particles are typically 12 nanometers in diameter – 1000 ferritin particles placed side-by-side would be as wide as a human hair. Ferritin may have first appeared during the Great Oxygenation Event when the interaction of ferrous iron with hydrogen peroxide in cells led to the generation of damaging reactive oxygen species (ROS) in cells and the need for cellular controls for ROS.
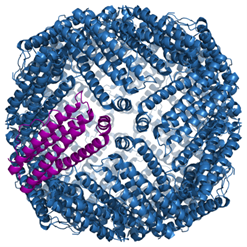
Figure 1 – Ferritin is a protein complex that forms from protein subunits that self-assemble into a hollow shell that is ~12 nanometers in diameter. Fe2+ ions enter through the pores between subunits, are oxidized to Fe3+ by ferroxidase centers and are stored as SPIONs.
One of the first scientists to investigate the quantum biological properties of ferritin in vitro (Latin for “in glass”) was Prof. Gary Watt, who observed in the 1980s that electron tunneling might be involved with iron storage. Over the next several decades, it was discovered that ferritin in vitro can absorb UV photons and store that energy as electrons in the core of ferritin. The interaction of photons with chemicals is inherently quantum mechanical, and was one of the first applications of QM to biological systems. Individual ferritin particles also support electron tunneling under ambient conditions over distances as great as 12 nanometers. This early work identified problems with ferritin that classical approaches could not solve, but which QM does.
What is tunneling in the context of QM? Electron and proton tunneling are the ability for those subatomic particles to pass through barriers that would be physically impossible under classical models, which typically occurs when a particle is “coherent” for a sufficient time for the probability wave function of the particle in free space to describe its behavior. “Hopping” is closely related to tunneling, and does not require the wavefunction of a particle to be “coherent” but which is nonetheless explained by the way that the particle wavefunction interacts with the wavefunctions of other particles. Interacting particle wavefunctions are very difficult to model mathematically because they depend on material-specific configurations, but the collective excitations of subatomic particles are often manifested as “quasiparticles,” which are not actual particles but which appear to have particle-like behavior. One example of a quasiparticle is an exciton, which is an electron and proton wavefunction that are entangled. The behavior of an exciton does not necessarily require the same electron and proton to remain entangled, and an exciton can define the movement of energy through a material like the way that sound waves define the movement of sound energy through materials. In both cases, it is the energy that is moving and not the particles.
Regarding electron tunneling, ferritin behaves like a quantum dot (QD), a man-made semiconductor device that has nanometer dimensions and which can spatially isolate electrons and allow them to exhibit QM properties such as electron tunneling and quasiparticle formation. Significantly, QDs (like ferritin) and pi-conjugated polymers (similar to melanin) have been used in solar photovoltaic devices to convert photon energy into electrical energy and to conduct that electrical energy to metal terminals via excitons. But it was not until 2018 that the first quantum biological function for these unusual biophysical properties of ferritin was hypothesized, namely, that ferritin could provide a medium for electrons to move by sequential tunneling between SNc and LC neuron soma over distances of 20 microns or more, and that those QM properties could also provide a mechanism for routing that energy (3). This hypothesized signaling mechanism could contribute to calcium signaling by causing the release of iron inside of the cell bodies of those neurons, which stimulates calcium release from the endoplasmic reticulum through ryanodine receptors (4, 5), The ability for cellular iron to contribute to calcium signaling in neurons in this manner has been experimentally shown in vivo (Latin for “in a living organism”). This hypothesis also answers two major unanswered question in neuroscience – 1) how thousands of large SNc and LC neurons act together as switches for selecting actions, and 2) how vast amounts of highly processed neural information signals are integrated into the singular experience of consciousness.
Shortly after the hypothesis was published in 2018, the first evidence of ferritin-mediated electron transport over distances of 40 microns was obtained, as predicted by the hypothesis (6). In 2019, evidence of widespread electron tunneling was obtained in fixed human SNc tissue, which was also predicted (7). In 2021, evidence of ferritin-mediated electron transport over distances of 80 microns was independently obtained, as well as a Coulomb-blockade routing mechanism like one that has been observed in QD structures from electron tunneling, both of which were predicted by the neural signaling hypothesis (8). Evidence of Coulomb blockade formation in ferritin was subsequently obtained independently. Thus, evidence was obtained of several important predictions that were made by the ferritin neural signaling hypothesis, and that evidence has been confirmed by independent tests.
Beyond Iron Storage: Evidence of Ferritin’s Unusual Biophysical Properties
Biologists understand that ferritin’s role in cells is to provide iron storage as part of the complex iron homeostasis system. It is a component of the labile iron pool in the cellular cytoplasm, which tightly regulates the availability of highly damaging iron for use in cellular processes. However, there are many unanswered questions about how ferritin can precisely store and release iron that classical approaches to analyzing ferritin cannot explain. In addition, ferritin is present in many cells at levels that are far greater than what is needed for iron homeostasis, sometimes to the point of damaging or killing the cell. The unique electrical, optical and magnetic properties of individual particles of ferritin are very different from what is observed when ferritin is studied in bulk (in large quantities that are compressed together), which has led to new hypotheses about its roles in biology and disease.
Electrical Properties: It is not possible to see electron tunneling, instead, it must be inferred by electrons behaving in ways that cannot be explained classically. Individual ferritin particles have been shown to store and conduct electricity in ways like QDs, which use the QM properties of electrons for those functions. Evidence in semiconductor devices shows that electrons can tunnel through semi-ordered arrays of ferritin particles that are like those seen in many different types of cells, like those shown in Figure 2, over relatively long distances for biological molecules. Moving electrical charges also generate magnetic fields, in accordance with the Biot-Savart Law, which may contribute to the observed magnetic properties of ferritin.
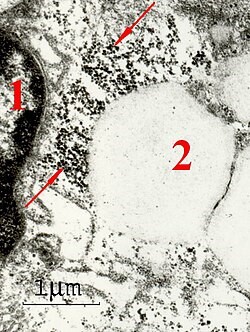
Figure 2 - Ferritin in a placental macrophage (red arrows) has aligned magnetic moments. The ferritin is small black electron-dense dots in this electron microscope image, which form array-like structures. Electron movement through these arrays could cause the alignment of magnetic moments through the Biot-Savart law, and remnant magnetization has been observed in these structures. 1- nucleus, 2 - vacuole.
Optical properties: As discussed, it has been shown in vitro that ferritin can absorb UV photons and can store that energy as an electron in its SPION core. In vivo, ferritin is overexpressed in skin cells in response to UV exposure, and is also present in the eye in concentrations greater than what is required for iron homeostasis. While very little UV penetrates the cornea, even low levels of UV over time could damage the sensitive optical detection system of the eye. Ferritin has also been shown to protect corneal epithelium cells in the eye from DNA damage. Ferritin’s quantum biological properties can explain why ferritin is present in skin and eye cells at levels that are greater than what is required for iron homeostasis.
Magnetic Properties: Ferritin is generally considered superparamagnetic in bulk, meaning it can be magnetized but doesn’t retain magnetization. However, at the nanoscale, individual ferritin particles can show ferromagnetic properties (retaining magnetization), which is unusual and not fully explained by traditional models of SPIONs in the core of ferritin. Research by Prof. Ron Naaman, one of the scientists who discovered a QM physical mechanism in chiral molecules known as chiral-induced spin selectivity (CISS), has shown that chiral molecules like the protein shell subunits of ferritin can “imprint” ferromagnetism on superparamagnetic iron oxide nanoparticles or SPIONs, like those found in the core of ferritin (9). These ferromagnetic properties are likely the result of collective excitations, such as a type of quasiparticle called a magnon. The magnetic properties of ferritin may be used by macrophages in avian magnetosensation systems to generate electron signaling that contributes to calcium signaling.
Ferritin’s Role in Health and Disease
In addition to the evidence discussed above, the quantum biological properties of ferritin appear to be implicated in numerous different biological systems and processes. While additional testing is needed to confirm these hypotheses, the circumstantial evidence of ferritin’s involvement is strong.
Antioxidant Function: Free radicals and ROS are chemicals in cells that can receive an electron from (oxidant) or donate an electron to (reductant) other chemicals in cells. While normal cellular processes use low levels of free radicals and ROS, excessive levels of those chemicals can damage cells. Antioxidants are reductants that can neutralize oxidizing free radicals.
Ferritin is overexpressed by cells and cellular systems in response to inflammation, such as cancer (10), which can be caused by excessive free radicals and ROS. Although ferritin may have initially evolved as an iron storage protein complex, ferritin has been shown to act like an antioxidant in vivo to neutralize harmful levels of oxidants, and to neutralize reductants by accepting the electron from them. It has also been shown by experiments to buffer electrons from antioxidants for hours, thus extending the antioxidant functional period. Its ability to act like a nanoscale ferromagnetic could allow it to attract and interact with paramagnetic molecules (those with unpaired electrons, which include some reductants and oxidants), which may be key to this antioxidant function. Overexpression of ferritin would not necessarily disrupt the labile iron pool of the cell, because the proteins and enzymes of the labile iron pool control the way iron is stored and released from ferritin. Cells that overexpressed ferritin in response to excess free radicals and ROS would have survived under conditions that killed cells without that capability. While the quantum biology of these functions has not yet been verified by testing in vivo, the circumstantial evidence suggests that it is present because it would provide an answer to unanswered questions about why ferritin is overexpressed in response to inflammation. According to the classical understanding of ferritin, it should cause inflammation by overloading a cell with iron when it is overexpressed, but that does not happen.
Neuron function: Myelin is a component of many neurons that have long axons and dendrites, and it supports a special type of neural signal conduction called saltatory conduction. Ferritin is present in myelin in quantities that are more than what is needed for iron homeostasis and could provide support for a recently discovered periaxonal nanocircuit in myelin that was not only predicted by classical models of myelin, but which explains the electrical behavior of myelin that classical approaches cannot explain (11). Myelin is formed around neuron axons and dendrites by oligodendrites and Schwann cells, which are phagocytes like macrophages, and which have some of the highest concentrations of ferritin of all cells.
Brain Function: Ferritin is found in high concentrations in certain brain cells, especially those involved in movement and dopamine/norepinephrine production. Dopamine neurons with high levels of ferritin and movement-related functions are found in simple animals at the base of the evolutionary tree, like the nematode C. elegans. Its interaction with melanin may be important for signaling and protecting neurons. Disruption of these interactions could contribute to diseases like Parkinson’s. In Figure 3, iron that is likely in ferritin is shown by electron spectroscopic imaging located outside of neuromelanin organelles (NMOs). In Figure 4, conductive atomic force microscopy tests are consistent with electron tunneling in layers of ferritin outside of 300 nanometer round structures that correspond to NMOs.
More significantly, the hypothesized ferritin-based signaling mechanism can provide a binding mechanism that is a neural correlate of consciousness (NCC), and which is consistent with other consciousness theories based on NCCs, such as the Integrated Information Theory and the Global Neuronal Workspace Theory (12, 13). Strongly correlated electrons in ferritin-based signaling structures like those found in SNc and LC neurons have wavefunctions that are entangled and could integrate information from the cortical and sensory neural signals that are provided to those neurons for action selection.
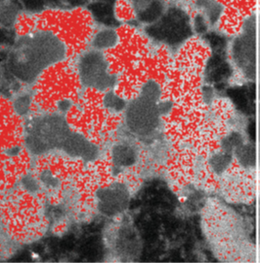
Figure 3 - Iron is shown in red outside of NMOs in the SNc, in this electron spectroscopic image, demonstrating the interaction of ferritin and neuromelanin in SNc neurons. From Sulzer, David, et al. "Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease." NPJ Parkinson's disease 4.1 (2018): 11.
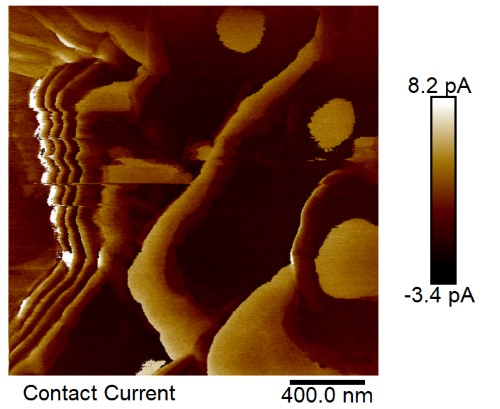
Figure 4 – The positive measured currents are an indication of electron tunneling from conductive atomic force microscopy tests on SNc neural tissue, because classical electron behavior would only produce negative currents. The location of these positive currents corresponds to layers of ferritin outside of NMOs.
Discussion
Quantum physics explains the behavior of individual fundamental particles like electrons and photons under biologically relevant conditions. QM also explains how fundamental particles interact with each other, although many of those interactions can be explained in a more general manner using classical descriptions of that behavior. Where classical approaches fail to provide explanations is often where quantum biology steps in. One effective approach for investigating quantum biology, using ferritin as an example, is to first identify the potential quantum biological mechanisms of interest (for ferritin, those are electron tunneling, electron storage and UV absorption), and then look for unanswered questions from biology that classical approaches cannot solve (for ferritin, those are its antioxidant function and overexpression in tissues beyond what is needed for iron homeostasis). That can occur at the level of individual particles, molecular structures or biological systems (for ferritin, see above). Many of these unanswered questions exist for single celled organisms and simple multicellular animals, and the quantum biological solutions that evolved for those simpler organisms were maintained and expanded in more complex organisms.
Experimentally investigating quantum biology can be difficult. While in vitro testing can provide some evidence of quantum biological functions and is possibly a starting point for identifying and understanding in vivo behavior, the cellular environment is complex and contains a wide variety of chemical compounds with dynamic behavior. It is important to design experiments for quantum biology that can be performed in vivo, and the best experiments should explain biological processes that cannot otherwise be explained using classical approaches. Hypothesized quantum biological processes that propose solutions to problems that do not exist, and which cannot be experimentally verified cannot distinguish fact from fancy.
For example, light-activated biochemical processes are inherently quantum biological, because photons are quanta of energy. Photons at certain energy levels can interact with specific chemical compounds to cause chemical reactions but will simply be absorbed and re-emitted by other chemical compounds as new photons. Whether these processes make use of long-lived quantum coherence depends on how you define “long-lived” and “quantum coherence.” The interaction of all fundamental particles is defined by the way that their probability wavefunctions interact with each other, and even without long-lived quantum coherence, particles can still be entangled in ways that form quasiparticles. Tests for avian magnetosensation mechanisms have shown that photons alone do not explain that phenomenon, simply by tracking migrating birds at night and conducting other experiments in the dark.
Coherence and decoherence in the cellular environment are only part of the analysis, and the behavior of quasiparticles in the cellular environment is of equal or possibly greater importance than coherence for some processes. Radical pairs of electrons and pigment-protein complexes can generate excitons, and the magnetic behavior of ferritin may result from magnons. Electron hopping involves entanglement but not coherence, and quantum biological electron transport could involve sequential tunneling and hopping processes. Advances in quantum thermodynamics are relevant to a type of quasiparticle called a phonon and may also help to analyze both the movement of other quasiparticles like excitons and information flow in the strongly correlated electron structures in SNc and LC neurons, which are modulated by billions of time-varying neural signals that represent cognitive information.
Conclusion
The investigation of quantum biology requires an open mind, and evaluation of unusual physical properties of biological compounds and molecules. Using ferritin as an example, while it is an iron storage protein with well-known classical functions that do not require QM to understand, its unique electrical and magnetic properties at the nanoscale may be crucial for many cellular processes, including protection against oxidative stress, neural signaling, and sensing magnetic fields. These discoveries open new avenues for research and potential therapies for a range of diseases. Understanding ferritin’s full range of functions could have significant implications for medicine, biology, and even technology. Similar approaches may apply to identify quantum biological processes that use radical pairs, pigment-protein complexes or other unusual physical properties of biological compounds.
(1) Busch, Andreas, et al. "Ferritin is required for rapid remodeling of the photosynthetic apparatus and minimizes photo‐oxidative stress in response to iron availability in Chlamydomonas reinhardtii." The Plant Journal 55.2 (2008): 201-211.
(2) Perez ID, Lim S, Nijhuis CA, Pluchery O, Rourk CJ. Electron tunneling in ferritin and associated biosystems. IEEE Transactions on Molecular, Biological, and Multi-Scale Communications. 2023 May 12;9(2):263-72.
(3) Rourk CJ. Ferritin and neuromelanin “quantum dot” array structures in dopamine neurons of the substantia nigra pars compacta and norepinephrine neurons of the locus coeruleus. Biosystems. 2018 Sep 1;171:48-58.
(4) Hidalgo C, Núñez MT. Calcium, iron and neuronal function. IUBMB life. 2007;59(4‐5):280-5.
(5) Guan, Wenzheng, et al. "Iron induces two distinct Ca2+ signalling cascades in astrocytes." Communications biology 4.1 (2021): 525.
(6) Bera S, Kolay J, Pramanik P, Bhattacharyya A, Mukhopadhyay R. Long-range solid-state electron transport through ferritin multilayers. Journal of Materials Chemistry C. 2019;7(29):9038-48.
(7) Rourk CJ. Indication of quantum mechanical electron transport in human substantia nigra tissue from conductive atomic force microscopy analysis. Biosystems. 2019 May 1;179:30-8.
(8) Rourk, Christopher, et al. "Indication of strongly correlated electron transport and Mott insulator in disordered multilayer ferritin structures (DMFS)." Materials 14.16 (2021): 4527.
(9) Koplovitz G, Leitus G, Ghosh S, Bloom BP, Yochelis S, Rotem D, Vischio F, Striccoli M, Fanizza E, Naaman R, Waldeck DH. Single domain 10 nm ferromagnetism imprinted on superparamagnetic nanoparticles using chiral molecules. Small. 2019 Jan;15(1):1804557.
(10) Alkhateeb AA, Han B, Connor JR. Ferritin stimulates breast cancer cells through an iron-independent mechanism and is localized within tumor-associated macrophages. Breast cancer research and treatment. 2013 Feb;137(3):733-44.
(11) Cohen CC, Popovic MA, Klooster J, Weil MT, Möbius W, Nave KA, Kole MH. Saltatory conduction along myelinated axons involves a periaxonal nanocircuit. Cell. 2020 Jan 23;180(2):311-22.
(12) Rourk C. Application of the catecholaminergic neuron electron transport (CNET) physical substrate for consciousness and action selection to integrated information theory. Entropy. 2022 Jan 6;24(1):91.
(13) Comment by Rourk, Christopher to Albantakis, Larissa, Robert Prentner, and Ian Durham. "Computing the integrated information of a quantum mechanism." Entropy 25.3 (2023): 449.